|
|
[QUE/TH-06007] TH-PROBLEMNode id: 5180page
|
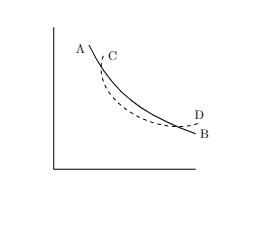
Consider a P-V diagram for a system as shown below
Assume the solid curve AB is isothermal ( system in thermal contact with a reservoit at constat temperature) and the dashed curve CD is adiabatic. Show that they can not intersect at two points as shown.
|
 |
|

|
22-01-23 19:01:46 |
n |
|
|
[QUE/TH-08009] TH-PROBLEMNode id: 5220pageFor a system with two coordinates $PV$ and two other
thermodynamic coordinates $X,Y$
$$ dW = -PdW+YdX $$
a process from state 1 to state 2 at constant temperature and
constant $X$ is possible only if
\begin{eqnarray}
G_1 \le G_2\qquad&\qquad G_1\ge G_2&
\end{eqnarray}
|

|
22-01-23 18:01:52 |
n |
|
|
[QUE/TH-08008] TH-PROBLEMNode id: 5219pageFor a system, as in $Q[2]$, undergoing a process from state 1
to state 2 at constant pressure and temperature, show that the
maximum ``non'' $PdV$ work out put is given by
\begin{eqnarray}
|A_{TP}| \le G_1-G_2 &\qquad G=\text{Gibbs function}&
\end{eqnarray}
|

|
22-01-23 18:01:29 |
n |
|
|
[QUE/TH-08007] TH-PROBLEMNode id: 5218pageFor a two component system with coordinates $P,V,X,Y$
($dw=-PdV+YdX$) show that the maximum workout put at constant $V$ &
$T$ is less than or equal to the decrease in free energy
($F_1-F_2$).
|

|
22-01-23 18:01:01 |
n |
|
|
[QUE/TH-08006] TH-PROBLEMNode id: 5217page\noindent (a)~ Consider a PV system undergoing change of state from 1 to
2. If the system is in contact with a thermal reservoir at
temperature $T$, show that the maximum amount of work out put
$|W_0|$ is given by
\begin{eqnarray}
|W_0| \le& F_1-F_2&\hfill{[4]}
\end{eqnarray}
\noindent(b)~For a reversible process show that
$$ |W_0| = F_1-F_2 $$
where $F$ is the Helmholtz free energy \hfill{[4]}
|

|
22-01-23 18:01:54 |
n |
|
|
[QUE/TH-07010] TH-PROBLEMNode id: 5216pageThe temperature of a household refrigerator is 5$^\circ$C and
the temperature of the room in which it is located is 20$^\circ$C.
If the refrigerator is to be kept cold heat of about $3\times10^8$ J
must be pumped out every 24 hrs. If the refrigerator is 60\% as
efficient as a Carnot engine operating between reservoirs having the
temperatures 5$^\circ$C and 20$^\circ$C, how much power in Watts
will be required to operate it?
{\bf NOTE:}In the last question it is not clear whether $3\times10^6$J is
absorbed at 5$^\circ$C or rejected at 20$^\circ$C.
|

|
22-01-23 18:01:52 |
n |
|
|
[QUE/TH-07009] TH-PROBLEMNode id: 5215pageTen grams of water at 20$^\circ$C is converted into ice at
-10$^\circ$C at constant atmospheric pressure. Assuming the heat
capacity per gram of liquid water to remain constant at 4.2 J/g\,K,
and that of ice to be one half of this value, and taking the heat of
fusion of ice at 0$^\circ$C to be 335 J/g, calculate the total
entropy change of the system.
|

|
22-01-23 11:01:19 |
n |
|
|
[QUE/TH-07008] TH-PROBLEMNode id: 5214pageA cylinder closed at both ends with adiabatic walls, is divided into two parts by a movable piston. The piston is frictionless and adiabatic. Originally, the pressure, volume, and the temperature of the gas are the same, $(P_0,V_0,T_0)$, on the two sides of the piston. The gas is ideal gas with $C_v$ independent of $T$ and $\gamma=1.5$. By means of a heating coil on the left hand side, heat is slowly supplied to the gas on the left hand side until the pressure reaches ${27\over 8}P_0$.
- what is the entropy change of the gas on the left?
- what is the entropy change of the gas on the right?
|

|
22-01-23 11:01:17 |
n |
|
|
[QUE/TH-07007] TH-PROBLEMNode id: 5213page A mass $m$ of a liquid at a temperature $T_1$ is mixed with an
equal mass of the same liquid at a temperature $T_2$. The system is
thermally isolated. Show that the entropy change is given by
$$
2m\,C_p\,\ln\left({(T_1+T_2)/2\over\sqrt{T_1T_2}}\right)
$$
and prove that this is necessarily positive.
|

|
22-01-23 11:01:59 |
n |
|
|
[QUE/TH-07006] TH-PROBLEMNode id: 5212page An inventor claims to have developed an engine that takes in
$10^7$ J at a temperature of 400 K, rejects $4\times10^6$J at a
temperature of 200 K and delivers $3.6\times10^6$J of mechanical
work. Would you advise investing money to put this engine on the
market? WHY?
|

|
22-01-23 11:01:53 |
n |
|
|
[QUE/TH-07005] TH-PROBLEMNode id: 5211page Liquid water having a mass of 10 kg and a temperature of
20$^\circ$C is mixed with 2 kg of ice at a temperature of
$-5^\circ$C at 1 atm pressure until equilibrium is reached. Compute
final temperature and the change in entropy of the system
$ C_p\,\text{(water)}=4.18\times10^3 {\rm K}^{-1}{\rm kg}^{-1}~;~~
C_p{\rm (ice)}=2.09\times10^3{\rm J}\,{\rm kg}^{-1}{\rm K}^{-1}~;~
l_{12}=3.34\times10^5{\rm J}\,{\rm kg}^{-1}$
|

|
22-01-23 11:01:52 |
n |
|
|
[QUE/TH-07004] TH-PROBLEMNode id: 5210page A 50 $\Omega$ resistor carrying a constant current of 1 \AA is
kept at a constant temperature of 27$^\circ$C by a stream of cooling
water. In a time interval of 1 sec (a) what is the change in entropy
of the resistor (b) what is the change in entropy of the
universe.
|

|
22-01-23 11:01:59 |
n |
|
|
[QUE/TH-06014] TH-PROBLEMNode id: 5209page
- [(a)]~ Derive relations similar to $Pv^{\gamma}=$ const., $\theta v^{\gamma-1}=$ const. for a Van der Waals gas.\\
- [(b)]~ Compute the work done in a reversible adiabatic expansion by direct evaluation of $\int P dv$ and by the use of energy equation \begin{eqnarray} u = C_v\theta -~{a\over v}~+ \text{const.}&\hfill{[4+4]} \end{eqnarray}
|

|
22-01-23 11:01:56 |
n |
|
|
[QUE/TH-06013] TH-PROBLEMNode id: 5208pageAn ideal gas for which $C_v=3R/2$ is the working substance of
a cannot engine. During the isothermal expansion the volume doubles.
The ratio of the final volume to the initial volume in the adiabatic
expansion is 5.7. The work output of the engine is $9\times10^5$ J
in each cycle. Compute the temperature of reservoir between which
the engine operates. Number of moles $=10^3$
|

|
22-01-23 10:01:52 |
n |
|
|
[QUE/TH-06012] TH-PROBLEMNode id: 5207pageA cannot engine is operated between two heat reservoir of 400 K and 300 K
- [(a)] If the engine receives 122 Cal from reservoir at 400 K in each cycle, how many calories does it reject to the reservoir at 300 K.
- [(b)] If the engine is operated as a refrigerator and receives 1200 Cal from reservoir at 300 K, how many Calories does it deliver at 400 K
- [(c)] How much work is done by the engine in each case.
|

|
22-01-23 10:01:49 |
n |
|
|
[QUE/TH-06011] TH-PROBLEMNode id: 5206page(a) Show that the work done on an ideal gas to compress it
isothermally is greater than that necessary to compress it
adiabatically if the pressure change is the same in two
processes.
(b)~Show that the isothermal work is less than the adiabatic work if
the volume change is the some in two processes
(c)~As numerical example, compare the work done from initial
pressure and volume to be 10$^6$N/m$^2$ 0.5 m$^3$ kilomole$^{-}$ in
the isothermal and adiabatic process when
(i)~the pressure is doubled (ii)~ volume is halved.
|

|
22-01-20 15:01:42 |
n |
|
|
[QUE/TH-06010] TH-PROBLEMNode id: 5205pageThe specific internal energy of a Van der Waals gas is given by
$$
u=c_v-{a\over v}+\text{const}
$$
Show that
\begin{eqnarray}
c_p-c_v =& R{1\over1-{2a(v-b)^2\over R\theta v^3}}
\end{eqnarray}
|

|
22-01-20 10:01:28 |
n |
|
|
[QUE/TH-06009] TH-PROBLEMNode id: 5204pageIn the compression stroke of a Diesel engine, air is
compressed from atmospheric pressure and room temperature to about
${1\over 15}$ of its original volume. Find the final temperature,
assuming a reversible adiabatic compression.
|

|
22-01-20 10:01:18 |
n |
|
|
[QUE/TH-02012] TH-PROBLEMNode id: 5203pageIn the Fig.-2, let $P_2=10\times10^5$Nm$^{-2}$, $P_1=4\times10^5$Nm$^{-2}$, $v_1=2.5$m$^3$kilomole$^{-1}$. Find
- the temperature $T$,
- the specific volume $v_2$,
- the temperature at points $b$ and $d$,
- the actual volume $V$ at point $a$ if the system consists of 4 kilomoles of hydrogen,
- the mass of hydrogen.
|

|
22-01-20 09:01:47 |
n |
|
|
[QUE/TH-02011] TH-PROBLEMNode id: 5202pageFig.-2 shows five processes, $a-b,~b-c,~c-d,~d-a,~a-c$,
plotted in the $P-v$ plane for an ideal gas in a closed system. Show
the same processes (a) in the $P-T$ plane. (b) in the $T-v$
|

|
22-01-20 09:01:44 |
n |