$\newcommand{\DD}[2][]{\frac{d^2 #1}{d^2 #2}}$
$\newcommand{\matrixelement}[3]{\langle#1|#2|#3\rangle}$
$\newcommand{\PP}[2][]{\frac{\partial^2 #1}{\partial #2^2}}$
$\newcommand{\dd}[2][]{\frac{d#1}{d#2}}$
$\newcommand{\pp}[2][]{\frac{\partial #1}{\partial #2}}$
$\newcommand{\average}[2]{\langle#1|#2|#1\rangle}$
qm-lec-16006
Contents
- General Properties of Bound State Spectra
- Bound state spectrum
- Coulomb problem spectrum
- Accidental degeneracy
1. General Properties of Bound State Spectra.
A potential is spherically symmetric if in polar variables it depends only on $r$ and not on $\theta$ and $\phi$ coordinates . We shall now discuss general properties of solution of 3-dimensional Schr\"{o}dinger equation $ H\psi = E\psi $ where
$$H = {\vec{p}\,^2\over 2m} + V(r) $$
and the potential $V(r)$ is spherically symmetric.
Conserved quantities
We note that all the three components of $\vec{L}$ commute with Hamiltonian $$ {} [\vec{L} , H] = 0 $$ hence $$ {} [\vec{L}^2 , H] = 0\,\,\,. $$ The parity operator $\mathscr{P} $ $$ \mathscr{P} \psi(\vec{r}) = \psi(-\vec{r}) $$ also commutes with $L^2L_z$ and $H$, operators. Therefore, the eigen functions of $H$ will also be eigen functions of $L^2, L_z$ and parity and each level can be assigned a definite value of $l, m$ and parity. For a state with definite value of $l$, the value of parity is $\equiv(-1)^l$. In this case $L^2, L_z$ and $H$ form a complete commuting set. {$(2\ell+1)$ degeneracy } We use the notation $\ket{El,m}$ to denote the simultaneous eigenvector of $H, L^2$ and $L_z$ \begin{eqnarray} H\ket{E,l m} & = & E\ket{E,l m}\\ L^2\ket{E,l m} &=& l(l+1)\hbar^2\ket{E,l m}\\ \mbox{and}~~ L_z\ket{E,l m} &=& m\hbar\ket{E,l m}\\ \mathscr{P} \ket{E,l m} &=& (-1)^l\ket{E,l m} \end{eqnarray} Applying $L_-$ on $\ket{El,m}$ several times leads successively to \begin{eqnarray} \ket{E l, m-1>}~,~\ket{E,l,m-2} &\cdots& |E l, -l\rangle\\ \end{eqnarray} and the action of $L_+$ on $\ket{E,l m}$ leads to the states \begin{eqnarray} \ket{E l, m+1}~,~\ket{E,l,m+2} &\cdots& |E l, l\rangle \end{eqnarray}
All these states will have the same value of energy. This statement can be proved by making use of the fact that $H$ commutes with $L_\pm$ and that action of $L_+$ (or $L_-$) on $|Elm>$ leads to states $|El,m+1>$ (or $|El,m-1>$). Thus we see that the bound state energy eigenvalues of a spherically symmetric potential problem with have $(2l+1)$ fold degeneracy. ( What about the continuous energy eigenvalues? )
Radial wave function
The Schr\"odinger equation for a spherically symmetric potential can be solved by separation of variables in polar coordinates. The angular part of the wave functions turns out to be a spherical harmonic $Y_{lm}(\theta,\phi)$ and the wave function has the form $$ \psi(r,\theta,\phi) = R(r)Y_{lm}(\theta, \phi) $$ where $R(r)$ is radial wave function satisfying the Schr\"odinger equation $$ -{\hbar^2\over2m} {1\over r^2} {d\over dr} r^2{d\over dr} R(r) + \left(V(r) + {l(l+1)\hbar^2\over2mr^2}\right) R(r) = ER(r)\,\,\,. $$ If we define $\chi(r)=rR(r)$, the function $\chi$ satisfies the equation $$-{\hbar^2\over2m} {d^2\chi\over dr^2} + \left(V(r) + {l(l+1)\hbar^2\over2mr^2}-E\right)\chi = 0\,\,\,.$$ with boundary condition $\chi(r)|_{r=o}=0$, otherwise $R(r)$, the radial wave function will tend to $\infty$ as $r\to\infty$.
2. Bound state spectrum.
The equation for $\chi$ has the form of one dimensional Schr\"odinger equation. Let $n^{'}$ denote the number of zeros of the radial wave function, excluding $r=0$ and at $r=\infty$. Then for a fixed value of $l$, the energy will increase with $n^{'}$, $n^{'}=0,1,2,\cdots$ will correspond to, for a fixed $l$, the `ground' state, first excited state, the second excited state etc. Because of the $l$ dependence of the term ${l(l+1)\hbar^2\over2mr^2}$ in the potential appearing in equation for $\chi(r)$, we expect that as $l$ is changed, keeping the number of nodes to be the same, $E$ would also change. Increasing $l$ would lead to increase in $E$, when $n^{'}$ is kept fixed.
Thus the spectrum would appear as follows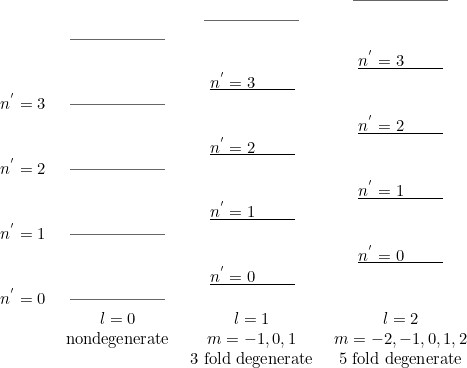
3. Coulomb problem spectrum
For hydrogen atom the energy levels are given by $$ E = -{Z^2
e^4m\over2\hbar^2(n^{'}+l+1)^2}$$ The energy does not depend on $n^{'}$
and $l$ separately but only on the combination $n=(n^{'}+l+1)$. For a fixed
$n$, $l$ can have values $0,1,\cdots,n-1$ ( because $n^{'}\ge0$ ) and all
these solutions correspond to the same energy eigenvalue. The energy level
diagram of H-atom, therefore, appears as shown below.

Putting all the levels which have the same energy together we get the following schematic representation of energy levels of $H$ atom. This table also shows that the allowed values of $l$ for each $n$, and number of $m$ values for each level. The number of total $m$ values, with the same energy, is $n^2$ and the degeneracy, after taking spin into account, becomes $2n^2$.
4. Accidental degeneracy.
Comparing the hydrogen atom levels with those of a general spherically symmetric potential, we find that energies for states with several different values of $l~ (=0,1,2 \dots n-1)$ are the same. For a general spherically symmetric potential different combinations of $n,l$ values correspond to different bound state energies, and are $(2l+1)$ fold degenerate. Thus there is an extra degeneracy is present for H atom beyond the expected $(2l+1)$ fold degeneracy this phenomenon present in the case of hydrogen atom is known as {\it accidental degeneracy.} Another well known case of accidental degeneracy is that of isotropic harmonic oscillator ( $ V(r)= {1\over 2} kr^2$) in three dimensions.
Remarks
- It must be emphasized that the accidental degeneracy is due to the special symmetry of the Coulomb problem.
- Any slight deviation of the potential from ${1\over r}$ will result in splitting of energy levels with different values of $l$.
- It is known that the accidental degeneracy is present whenever the Schr\"odinger equation $H\psi=E\psi$ can be separated into ordinary differential equations in more than one set of coordinate system. \noindent $H$ atom --- Separation of variables for the Coulomb problem is possible in
- spherical polar coordinates $r,\theta,\phi$
- parabolic coordinates $\xi, \eta, \phi$ defined by \begin{eqnarray} \xi = r-z = r(1-\cos\theta)\quad \eta = r+z =r(1+\cos\theta)\quad \phi = \phi \end{eqnarray}
- The isotropic oscillator also exhibits accidental degeneracy. For isotropic harmonic oscillator,$V(r) ={1\over 2} k r^2$, the Schrodinger equation can be separated in two set of variables, Cartesian and spherical polar coordinates.
Exclude node summary :
Exclude node links:
4727: Diamond Point, 4909: QM-HOME-I






 ||Message]
||Message]
